We have been looking after the health and lives of our patients since 1823
Drawing on 200 years of heritage, we are responding to the challenges of the future to ensure access to treatment for generations to come.
The beginnings of the formation of Polish chemical industry
In 1823, Ludwik Hirschmann and Jan Chryzostom Kijewski founded the Chemical Products Factory, the first enterprise in the Kingdom of Poland whose products were officially distributed to pharmacies.
The Tarchomin branch, established in 1826, initially focused on the production of food-grade vinegar and gradually expanded its operations to include chemicals, cosmetics, and pharmaceuticals.
This pioneering factory laid the foundations for Ludwik Spiess’s Chemical and Technical Works, which later developed into one of the largest pharmaceutical companies in Poland.
The future of the business in the hands of the Spiess family
In 1860, the factory in Tarchomin was taken over by the Spiess family, marking the beginning of a period of dynamic development. Production soon exceeded local demand, enabling exports to other European countries.
The company gradually increased its significance, transforming pharmaceutical manufacturing from a small-scale pharmacy–laboratory model into fully industrial production.
Over time, the product portfolio was expanded to include increasingly advanced pharmaceutical preparations. In 1875, the company commenced the production of medicinal galenical preparations, including plasters, tinctures, and herbal extracts.
Intensive expansion in the interwar period
In 1918, after Poland had regained its independence, the Tarchomin plant entered a period of intense development. Thanks to foreign capital, research and development facilities were expanded, making production independent of imported chemical raw materials.
Before World War II, the factory was the largest pharmaceutical manufacturer in Poland, producing 338 galenic preparations, 220 pharmaceuticals and 175 other products, including: Novarsenbenzol, Phosphit, vaccines, sulfonamide (Septazin), barbiturates, lanolin, vitamin D2 and Antiba cosmetics.
A time of difficult decisions
During World War II (1939-1945), production activities were significantly reduced as a result of the restrictions imposed by the occupying forces and difficulties with obtaining raw materials. The consequence was the cessation of production of Antiba cosmetics and a significant reduction in the workforce.
Despite the constant threat, many employees were actively involved in underground activities, fighting for free Warsaw and Poland, including in the Warsaw Uprising.
After the war, the factory, which had been destroyed and robbed of its equipment, was nationalised. The resumption of production – initially limited to simple ointments and galenical preparations – marked the beginning of the process of rebuilding the Polish pharmaceutical industry.
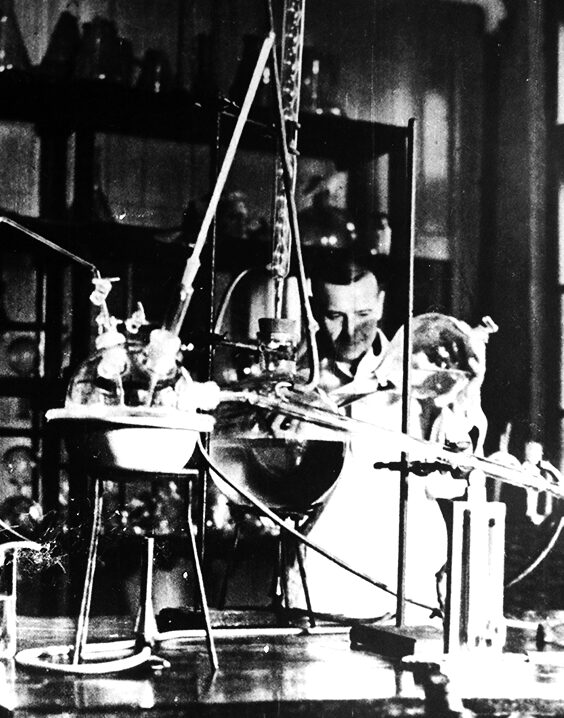
The first Polish penicillin
A major turning point after the Second World War was the decision to construct the first penicillin factory in Poland – one of the first in Europe – at the Tarchomin site.
In 1947, the knowledge required to initiate antibiotic production was transferred from Toronto through the efforts of two Polish scientists. Just one year later, in 1948, the first 4 grams of penicillin were successfully produced by a team of fifteen researchers working in a modestly equipped microbiology laboratory located in a building known as the “Mouse Tower”.
The resulting substance was yellow, amorphous, and sensitive to temperature; nevertheless, it constituted tangible proof that a promising future lay ahead for the plant.
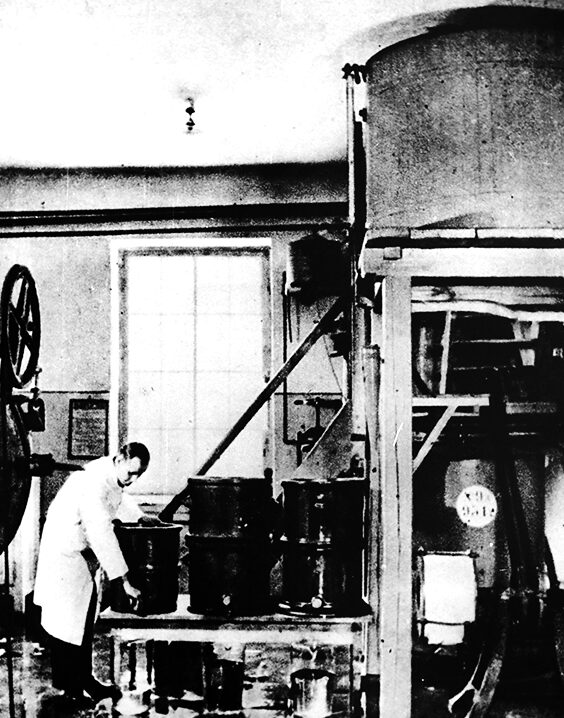
Start of insulin production
In 1953, the Tarchomin plant was one of the first in the world to start producing insulin.
Initially, it was amorphous insulin, but by 1958 conventional bovine and porcine insulin was being produced. Further development took place between 1965 and 1966, when technologies were developed to produce preparations with different kinetics of action. Advances in purification resulted in the introduction of chromatographically purified insulin (so-called ‘mono peak’) in 1986.
This consistent development, with the key contribution of Polish researchers, significantly increased the therapeutic options for the treatment of diabetes in the 20th century.
Leading antibiotic manufacturer
The year 1958 marked a key stage in the development and dissemination of antibiotic manufacturing, positioning the Tarchomin plant as a leading Polish manufacturer.
Instead of relying on imports, the company relied on its own biosynthesis. Apparatus was upgraded and new production buildings equipped with research and development laboratories were put into service.
Production began with oxytetracycline and streptomycin. The range was quickly expanded, however: in 1961, tetracyclines were introduced, followed a year later by erythromycin. This dynamic development has significantly strengthened the independence of Polish pharmacy.
Systemic changes and entry into the new millennium
The Tarchomin plant, operating under the name Polfa Tarchomin since 1960, successfully navigated the political transformation following the collapse of communism and the challenging economic conditions of the early 1990s by adapting to global production standards compliant with Good Manufacturing Practice (GMP) requirements.
Entering a new political and economic era, the company underwent a dynamic sequence of ownership changes. In 1994, it was transformed into a sole-shareholder company of the State Treasury; in 2004, it became part of Polski Holding Farmaceutyczny (PHF); and in 2013, it returned to direct State Treasury ownership as Tarchomińskie Zakłady Farmaceutyczne „Polfa” S.A.
Start of human insulin production
Since the late 1950s crystalline insulin of animal origin was produced in Tarchomin. In the following decades, technologies were developed to obtain preparations with varying times of action, resulting in the introduction of chromatographically purified insulin in 1986.
A new phase in the company’s history began in 2001, when work on human insulin production technology was undertaken. In 2005, its production was launched on an industrial scale, which significantly increased the availability of modern insulin therapy for patients.
Growth on international markets
TZF Polfa consistently strengthens its presence on international markets. A turning point came in 2018, when the company presented its range of products for the first time at CPHI fairs in Madrid.
Since then, it has regularly attended major industry events around the world, established new business relationships and developed contract manufacturing (CMO/CDMO) collaborations.
Today, TZF Polfa’s products are distributed to more than 60 countries on four continents and the company continues to strengthen its position internationally as a reliable partner for distributors and healthcare institutions.
Joint fight against the Covid-19 pandemic
TZF Polfa made a huge contribution to protecting human health and life during the COVID-19 pandemic. The production of disinfectant fluids, which were donated to hospitals and ambulance stations, was launched at an express pace. Medical oxygen has also been introduced to the market.
The plant then operated 24/7 and became one of the main sources of supply for the public sector. At this critical time, TZF Polfa has confirmed that it is a manufacturer that can be counted on to uphold quality, availability of therapies and medicines supply security for patients.
Start of construction of the Center for Development and Production of Highly Potent Drugs
In 2021, TZF Polfa initiated the construction of the Centre for the Development and Production of Highly Active Medicines – an investment unique both in Poland and in this part of Europe – implemented using state-of-the-art technologies.
The core of the new facility will comprise two production lines dedicated to the manufacture of highly potent oncology and immunosuppressive medicines (up to OEB 5), with an annual capacity of up to 20 million vials and 6 million ampoules.
The new plant will become a strategic hub for the development and production of advanced therapies, significantly strengthening national capabilities in the field of drug safety and pharmaceutical security.
"For External Use. Graphic Design in the Pharmaceutical Industry 1960-1980" Exhibition
In 2022, the history of TZF Polfa returned in a remarkable form thanks to an exhibition organised at the Museum of Warsaw.
Among the exhibits on display were leaflets, catalogues and other company materials from 1960-1980, which, despite their utilitarian character, impressed with their high artistic quality. The graphic designs from that period referenced the style of the Polish School of Posters, combining an informative function with aesthetics, making them unique testimonies of the era.
TZF Polfa not only contributed to the development of Polish pharmacy, but was also part of the broader movement of Polish graphic design culture.
TZF Polfa's 200th Anniversary
In 2023, TZF Polfa celebrated its 200th anniversary. The ceremonial gala was attended by guests from the pharmaceutical industry and representatives of state administration. A picnic was held at the company premises for employees, often representing successive generations building the company, which allowed them to jointly celebrate this historic anniversary.
The jubilee became an opportunity to honour the company’s rich tradition and its contribution to the development of Polish pharmacy, as well as a moment of reflection on the journey travelled over two centuries.
The celebrations coincided with the establishment of new strategic goals, focusing among other things on ensuring drug security and the production of preparations of crucial importance for patients’ health.
Ensuring the Availability of Medicines as a New Strategic Direction
In 2024, TZF Polfa has defined a new strategic direction, focusing on ensuring medicine availability in a dynamically changing economic environment.
The company currently produces 20 of the 32 items on the EU list of critical medicines that are generally produced in Poland. A portfolio based mainly on generic drugs, with a strong position in antibiotics, makes TZF Polfa one of the most important suppliers to the hospital and pharmacy markets.
Continuity of supply of these products is of direct relevance to the stable functioning of the healthcare system and strengthens patients’ medicine availability in real terms.
Our website features photos from the TZF Polfa archives. They show our former employees whose identities unfortunately we haven’t been able to identify, so we’re asking for your help. If you recognize yourself or someone in the footage, please contact us: pr@tzf.pl
Your information will be treated confidentially. Complete information about the processing of your personal data can be found in our Privacy Policy.